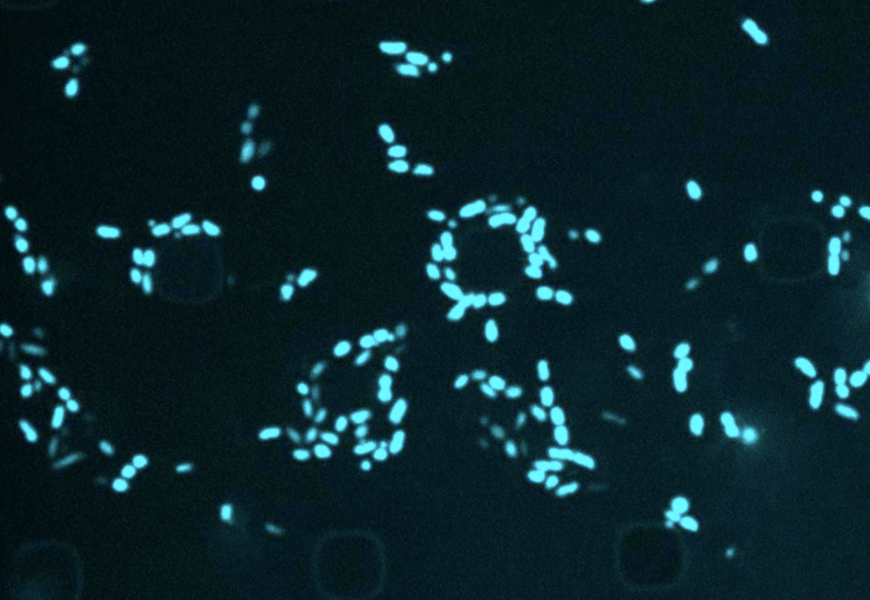
Modern antimicrobial discovery has stagnated since the 1970s, leading the World Health Organization to identify antimicrobial resistance as a significant global public health concern. The consistent use of antimicrobial treatments poses a risk as bacteria can develop resistance over time. This resistance may stem from bacteria entering a state of dormancy, rendering conventional medications ineffective against them until they become active again, causing the disease to resurface.
Jackie Valeri, a former MIT Takeda Fellow from the Collins Lab, recently obtained her PhD in genetic engineering and authored a report featured in Cell Chemical Biology. The study showcases how machine learning can assist in identifying latent bacteria that contribute to recurrent infections.
Recent discoveries have revealed ancient bacterial genotypes that have existed for millions of years in a dormant state at the bottom of the Pacific Ocean. This finding challenges the notion of bacterial resilience akin to “sleeper cells.”
James J. Collins, a Termeer Professor at MIT’s Institute for Medical Engineering and Science, gained recognition for utilizing AI to uncover a new class of medications. This breakthrough aligns with the team’s goal of leveraging AI to expand the range of available medications.
In 2019, a Lancet publication suggested that 1.27 million deaths could have been prevented if infections were drug-resistant. Researchers face the challenge of developing antibiotics that can effectively target metabolically dormant bacteria.
The Collins Lab researchers harnessed AI to expedite the discovery of antibiotics within existing drug compounds. Despite the typically time-consuming process of sifting through millions of molecules, the team identified semapimod within a weekend, showcasing AI’s efficiency in high-throughput screening.
Researchers found that semapimod, an anti-inflammatory drug used for Crohn’s disease, demonstrated efficacy against stationary-phase Escherichia coli and Acinetobacter baumannii. Furthermore, semapimod exhibited the ability to disrupt the membranes of Gram-negative bacteria, notorious for their resistance to antibiotics due to their robust outer membrane.
Gram-negative bacteria like E. coli, A. baumannii, Salmonella, and Pseudomonas pose challenges in developing new antibiotics. Semapimod’s unique structure enables it to sensitize Gram-negative bacteria to drugs effective against Gram-positive bacteria by targeting a component of the outer membrane.
Valeri references a 2013 Trends in Biotechnology paper, emphasizing the urgent need for effective drugs against Gram-negative infections.