This article is an on-site version of our Moral Money newsletter. Sign up here to get the newsletter sent straight to your inbox.
Visit our Moral Money hub for all the latest ESG news, opinion and analysis from around the FT
Over the past year or so, the rapid development of artificial intelligence has sparked trepidation over how it may disrupt various facets of the modern world — from job security to the development of new weapons systems.
But AI could also help businesses tackle some thorny, long-standing ethical challenges. Today I write about one of these potential areas of impact: efforts to replace animal testing in drug development. Thank you for reading.
ARTIFICIAL INTELLIGENCE
No more monkey business
Has anyone else noticed the exorbitant price of monkeys? Everything is just so expensive these days . . .
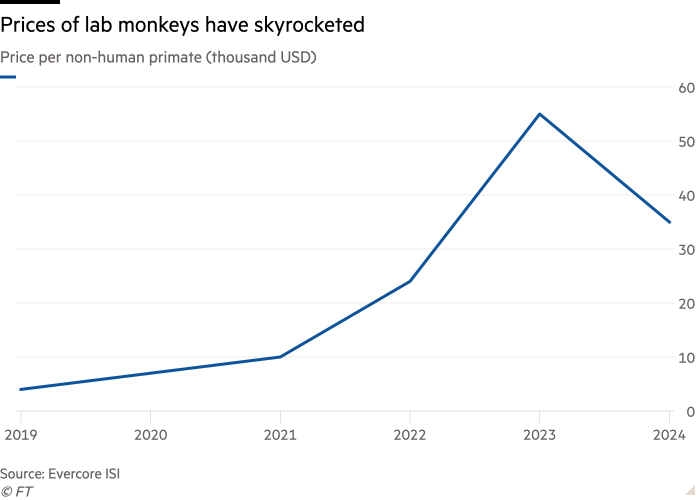
Jokes aside, prices of non-human primates (as they are referred to in the pharmaceutical industry) used to test treatments have been high since 2020, due to the halt of exports from China that stemmed from a push to build up the country’s biotech sector amid the Covid-19 pandemic.
In 2023, prices of these animals reached a peak of roughly $55,000, a more than tenfold increase from pre-pandemic levels, according to Elizabeth Anderson, senior managing director at Evercore ISI’s healthcare technology and distribution team.
At present, exports from China have not resumed, and prices remain high at around $35,000 to $40,000 per monkey.
Beyond these price pressures, pharmaceutical companies have other reasons to worry about their reliance on animal testing. For decades, they’ve faced shareholder proposals from organisations such as animal rights group Peta, alleging that the death and suffering of these animals is often unnecessary. Peta claims that these campaigns have pushed companies to invest in a range of alternative technologies.
Advances in artificial intelligence are now offering new opportunities to tackle this industry’s still massive reliance on animal testing. Quantiphi, an AI start-up based in Boston, embarked on a mission last year to build “digital animal replacement technology”, known as Dart.
The company has found that the technology can reduce both the time and cost of the pre-clinical testing phase of drug development by 45 per cent, Asif Hasan, co-founder of Quantiphi told me. Typically, this stage takes one to two years, costing around $120mn to $135mn per drug, with a heavy reliance on animal testing.
Currently, pharmaceutical companies often use animals such as monkeys, which are not only expensive but need to be exported from countries with warmer climates such as Cambodia, and can die in transit from illness or stress.
Many more animals perish during pre-clinical studies testing for toxicity, which in some cases require a postmortem examination of organs, before drugmakers are able to move on to clinical studies involving humans. Any animals still alive at the end of a trial are typically killed.
For example, when testing “one commercial batch of antivenom, you will utilise between 200 and 2,000 rabbits,” Rahul Ramchandra Ganar, principal lead at Quantiphi’s life sciences division, explained. Witnessing the deaths of these animals puts a major mental strain on scientists as well, he noted.
The reliance on animal testing brings further risks to the human subjects of clinical trials, and to the drug development process as a whole, Quantiphi argued.
“Animals do not resemble human physiology. And hence, when you go for clinical trials, there are many new adverse events or toxicology that get discovered,” Ganar said. The company works with Transcell, one of the world’s largest biobanks, and ethically sources its stem cells — by using freely donated umbilical cords, which Ganar said provides the purest form of stem cell.
To test a drug, Quantiphi first administers the drug to human cells. Then a digital microscope takes images which the AI system can use to analyse and count the number of dead and living cells. That analysis will help test for things like hepatotoxicity or cardiotoxicity — sources of potential injury to the liver or heart. Hasan tells me that the technology can achieve 92 to 95 per cent of the same results as traditional animal testing methods.
Quantiphi is working towards getting its first treatment using Dart to be approved by the US Food and Drug Administration. Over the long term, company executives hope its technology can contribute to cures for major diseases, including cancer. “We want to build the momentum, then we want to go after moonshots”, Hasan said.
There are a number of other players seeking to harness the power of AI to replace animal testing. One example is VeriSIM Life, a US-based biotech start-up that is using AI to create digital simulations that predict how a drug would react in both animal and human bodies.
Traditional healthcare companies are also striving to make headway in this space. The chief executive of Charles River Laboratories, a company that specialises in pre-clinical and clinical studies, recently told investors that they are “spending a lot of time appropriately looking at reducing non-human primate usage,” and over the past few years have invested $200mn on alternatives such as AI.
Crucially, regulators have been moving to encourage development in this space. A US law passed in 2022 eliminated the requirement for animal testing and allowed for digital alternatives. Now, the likes of Quantiphi will need to prove that these alternative technologies are truly viable. (Kaori Yoshida, Nikkei)
Smart read
Climate concerns have put low-carbon nuclear energy back in vogue. But supply chain disruptions, technical challenges and skills shortages are holding the sector back, write Rachel Millard and Jana Tauschinski.