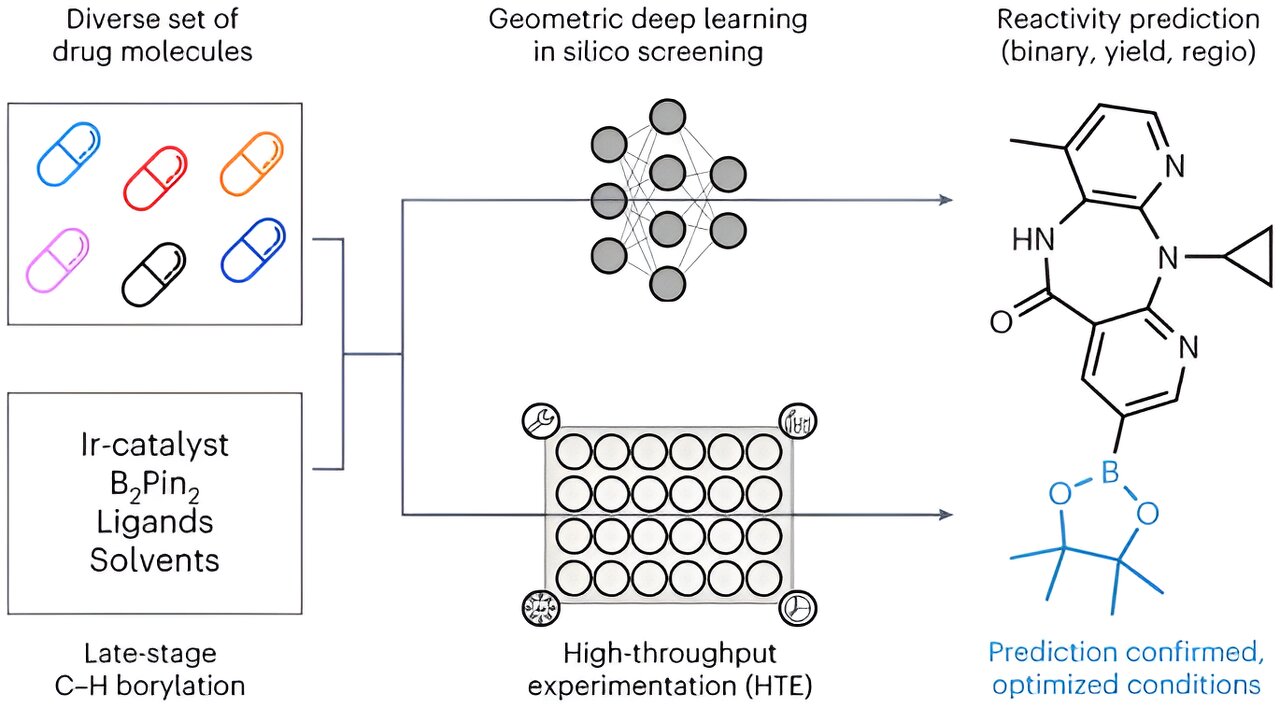
The cornerstone of innovative and superior medical therapies is established by fresh active pharmaceutical components. Nevertheless, the discovery and, more notably, the synthesis of these compounds through experimental chemical processes pose a formidable challenge. Typically, researchers utilize a trial-and-error approach to identify the optimal manufacturing method: they deduce potential laboratory production techniques from known chemical reactions and subsequently assess each one through experiments—a time-consuming process riddled with dead ends.
A revolutionary method developed by scientists from Roche Pharma Research and Early Development and ETH Zurich now allows for the determination of the most effective production process, along with its likelihood of success. Their findings have been documented in the Nature Chemistry journal.
Kenneth Atz, a doctoral candidate at the Institute of Pharmaceutical Sciences at ETH Zurich who collaborated with Professor Gisbert Schneider on Artificial design, asserts that “our strategy has the capacity to significantly diminish the number of required lab tests.”
Effective pharmaceutical components typically exhibit a scaffold with attached functional groups. These functional groups confer specific biological functions to the material. To ensure the precise operation of these functional groups, the scaffold’s role is to govern their spatial arrangement. Imagine a construction set for a crane where the driver’s cab, wheels, cable winches, and other functional components are accurately positioned relative to each other, thanks to the supporting framework of elements securely fastened together.
Illuminating the Chemical Procedures
One approach to developing medications with a novel or enhanced therapeutic impact involves introducing functional groups to new locations on the scaffolds. While this may appear simple in theory, chemistry presents substantial hurdles. This is primarily due to the composition of the scaffold, which primarily comprises carbon and hydrogen atoms, making it challenging to bond them with crucial atoms like oxygen, nitrogen, or chlorine. Activation through specific reactions is essential to biologically activate the scaffold.
Borylation emerges as a promising activation method that provides a plethora of options for integrating diverse functional groups. In this technique, a carbon atom within the scaffold is connected to a chemical group containing the carbon element. This carbon group can then be easily substituted with various functional groups recognized for their effectiveness in treating illnesses.
Insights from Trustworthy Sources and Computational Testing
Despite its potential, controlling the borylation process in the laboratory can be arduous. Consequently, Atz mentions that their extensive literature review uncovered slightly over 1,700 medical papers on the topic.
Their approach involved leveraging the insights derived from medical literature to train an AI model capable of generating new molecules and identifying potential borylation sites. However, the research team chose to utilize a specific subset of 38 highly credible publications to avoid erroneous conclusions stemming from inadequate analysis. This refined dataset encompassed a total of 1,380 interactions involving borylation.
In addition to the literature findings, the team integrated data from 1,000 reactions conducted in Roche’s automated laboratory for pharmacological chemistry to enrich their training dataset. This integration enabled the simultaneous evaluation of multiple biochemical reactions on a significant scale.
David Nippa, a graduate student from Roche involved in the project alongside Atz, emphasizes the vast potential of merging laboratory techniques with AI to boost efficiency in chemical synthesis while advocating for sustainability.
Enhanced Predictive Capabilities, Particularly with 3D Data
The predictive model derived from this amalgamation of information was validated using six well-known drug compounds. Empirical laboratory tests confirmed the model’s precision in predicting additional borylation sites in 5 out of 6 scenarios. Furthermore, the model efficiently pinpointed optimal conditions for activating these sites where detection was previously unattainable.
Significantly, the inclusion of 3D information regarding the starting materials, rather than solely relying on 2D calculations, further bolstered the accuracy of the predictions. Atz proposes that this approach furnishes the model with a nuanced, three-dimensional comprehension of chemical interactions.
The experts at Roche Pharma Research and Early Development expressed contentment with the model’s success rate, as it facilitated the identification of sites in existing medications where new functional groups could be introduced. This streamlined process expedites the development of potent variations of established active pharmaceutical ingredients.
Future Applications and Functionalizations
Atz and Schneider envision numerous potential applications for AI models based on a fusion of reliable sources and automated laboratory experiments. This methodology holds promise for developing efficient models for diverse reactions beyond borylation. Additionally, the team aims to explore a broader spectrum of reactions to further enhance the functionalization of borylated sites.
Currently, Atz is actively engaged in advancing this research as an AI scientist specializing in therapeutic chemical studies at Roche. The convergence of laboratory automation and scientific AI research presents an exciting frontier for exploration. Atz expresses his eagerness to propel this field forward using state-of-the-art strategies and resources.
Schneider underscores the importance of public-private partnerships in Switzerland, citing this project as a prime example of successful collaboration between academia and industry.