By leveraging quantum science and observable artificial intelligence, scientists at Oak Ridge National Laboratory have enhanced CRISPR Cas9 systems for modifying bacterial genomes. This breakthrough enhances the potential for chemical and alternative fuel production by enabling more precise genetic modifications in microbes.
The efficacy of CRISPR Cas9 DNA editing in microbes has significantly advanced through Oak Ridge National Laboratory’s research in classical biology and AI, contributing to solar energy development. Researchers at Oak Ridge National Laboratory (ORNL) have refined the functionality of CRISPR Cas9 genome editing tools on organisms such as microbes, which can be manipulated to produce sustainable fuels and chemicals. This improvement was achieved by harnessing their expertise in quantum biology, artificial intelligence, and bioengineering.
CRISPR, a powerful genetic engineering tool, can be utilized to modify genetic codes to enhance an organism’s performance or induce specific mutations. The CRISPR Cas9 system relies on a unique guide RNA for its operation.
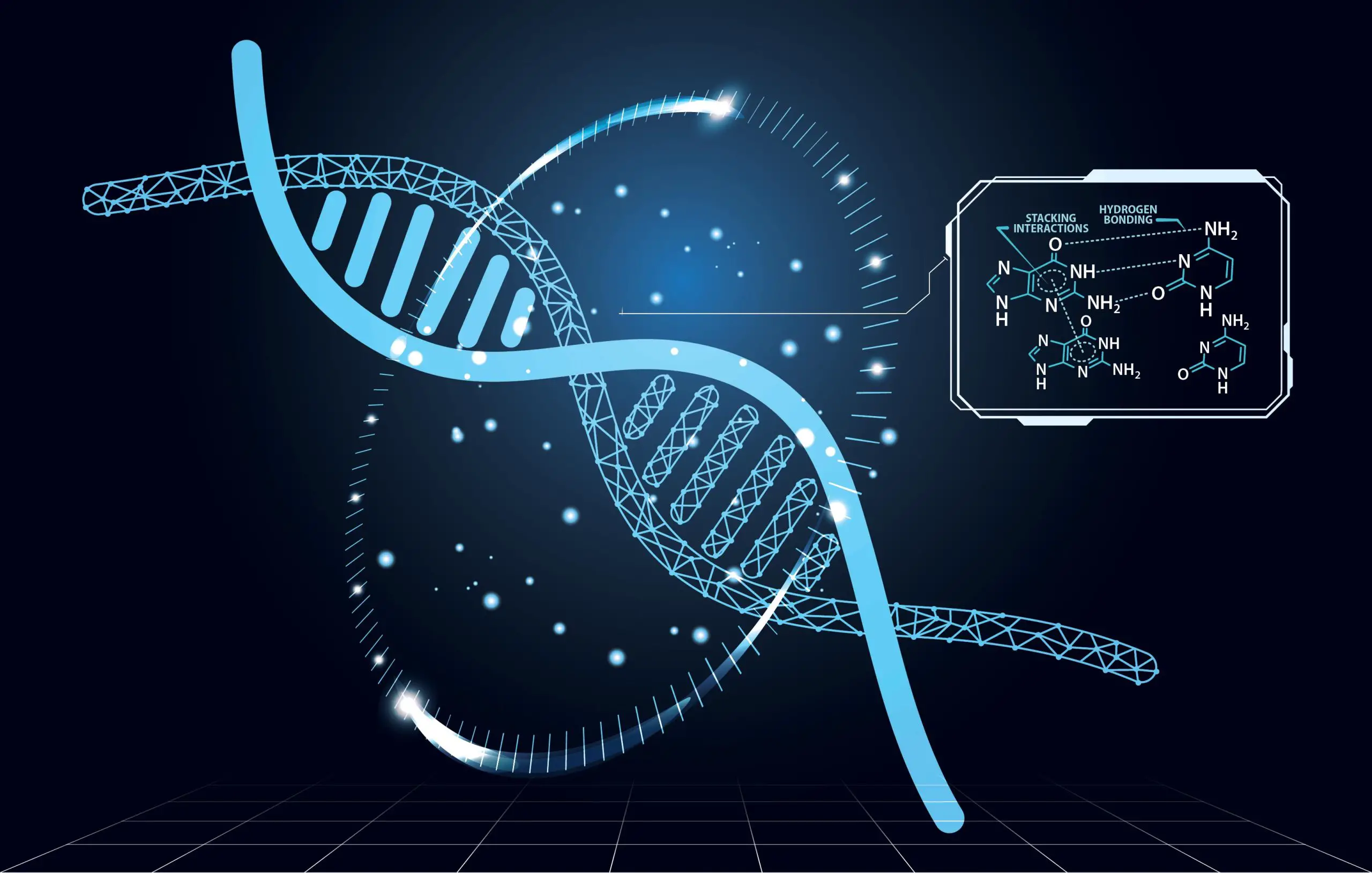
Ribonucleic acid (RNA) is a vital polymeric molecule essential for gene regulation, expression, and coding in various biological processes, distinct from DNA. RNA is single-stranded, unlike DNA, and consists of alternating sugar (ribose) and phosphate groups forming its backbone. Each sugar is linked to one of four bases: adenine (A), uracil (U), cytosine ©, or guanine (G). Within the cell, different types of RNA exist, including ribosomal (rRNA), messenger (mRNA), and transfer (tRNA) RNA.
The RNA responsible for guiding the Cas9 protein to bind and cleave the targeted site in the genome is referred to as “guide RNA.” Existing models were developed using limited data variants to computationally identify effective guide RNAs for CRISPR applications.
A species is a group of organisms capable of reproducing and producing fertile offspring with shared characteristics. The concept of species is fundamental in science for categorizing and managing biodiversity. The natural kinds theory defines a species as a population of animals capable of interbreeding and producing viable offspring in the wild. This definition is widely employed in evolutionary science and ecology to classify living organisms.
When applied to microbes, the efficiency of CRISPR tools varies significantly, highlighting the need for tailored approaches.
Focused CRISPR Research on Microbes
Numerous RNA tools have been developed for various organisms, but ORNL researchers, led by Carrie Eckert, have focused on microbes due to their unique genome structures and sizes. This study confirms the distinct response of CRISPR Cas9 technology when applied to microbes, emphasizing the importance of species-specific considerations.
The enhancement of CRISPR Cas9 DNA editing for microbes to facilitate chemical and alternative fuel production was a key achievement by ORNL researchers, drawing on their expertise in synthetic biology, artificial intelligence, and quantum biology.
To gain deeper insights into cellular processes at the molecular level, particularly within cell nuclei where genetic material is housed, researchers aimed to improve the modeling and design of guide RNAs. By exploring the impact of digital structure on the chemical properties and interactions of nucleotides, the building blocks of DNA, they turned to quantum science, combining molecular biology with quantum mechanics.
DNA, or deoxyribonucleic acid, consists of two long nucleotide strands forming a double helix structure. It serves as the hereditary material in humans and most animals, carrying genetic instructions for growth, development, and reproduction. While DNA is predominantly located in the cell nucleus, a small amount can also be found in mitochondria, known as mitochondrial DNA or mtDNA.
Electron distribution within proteins influences structural stability and sensitivity, affecting the binding efficiency of the Cas9 enzyme-guide RNA complex with microbial DNA, as explained by Erica Prates, a computing systems scientist at ORNL.
Leveraging Explanatory AI in CRISPR Research
The researchers developed the interpretable artificial intelligence model, Continuous Obscure Forest, to enhance guide RNA design for targeting E. coli bacterial genomes. By training the model with 50,000 guide RNAs and incorporating quantum chemical properties, they identified crucial nucleotide features for selecting optimal guide RNAs. This approach provided a wealth of molecular insights to advance CRISPR technology and uncover the underlying mechanisms of guide RNA effectiveness.
Through experimental validation using CRISPR Cas9 cutting tests on E. coli bacteria with a diverse set of guides selected by the model, ORNL researchers confirmed the utility of the interpretable AI model.
Observable AI offers a transparent understanding of the biological processes influencing outcomes, unlike deep learning models that operate as “black box” algorithms, noted Jaclyn Noshay, a former computing systems researcher at ORNL and lead author of the study.
The interpretable AI model, with its comprehensive features and incremental nature, was trained using the Summit supercomputer at ORNL’s Oak Ridge Leadership Computing Facility (OLCF), a Department of Energy (DOE) Office of Science resource.
Eckert mentioned plans to collaborate with mathematical science experts at ORNL to further refine the bacterial CRISPR Cas9 model based on the study findings and data from laboratory experiments involving diverse bacterial species.
Advancing CRISPR Cas9 Tools for Diverse Species
Considering classical attributes opens avenues for enhancing Cas9 guide RNA designs across various species. This research has implications beyond microbial applications, extending to pharmaceutical developments where precise genome targeting is crucial.
Enhancing CRISPR Cas9 models provides a robust framework for linking genes to phenotypes, advancing functional genomics research. The study’s outcomes are relevant to the ORNL-led Center for Bioenergy Innovation (CBI) in enhancing bioenergy crop plants and optimizing carbon bacterial fermentation.
The study aims to enhance the precision and efficiency of DNA modifications across diverse organisms using CRISPR tools. This research marks a significant step toward understanding how to avoid costly errors in an organism’s genetic code, noted Paul Abraham, a bioanalytical chemist at ORNL leading the DOE Genomic Science Program’s Safe Ecosystem Engineering and Design Science Focus Area (SEED SFA) supporting the CRISPR research.
Co-authors of the study included researchers from ORNL, the University of Tennessee, Knoxville, Bayer, and the University of Queensland, with funding provided by the SEED SFA, CBI, and various DOE Office of Science programs supporting high-performance computing resources.