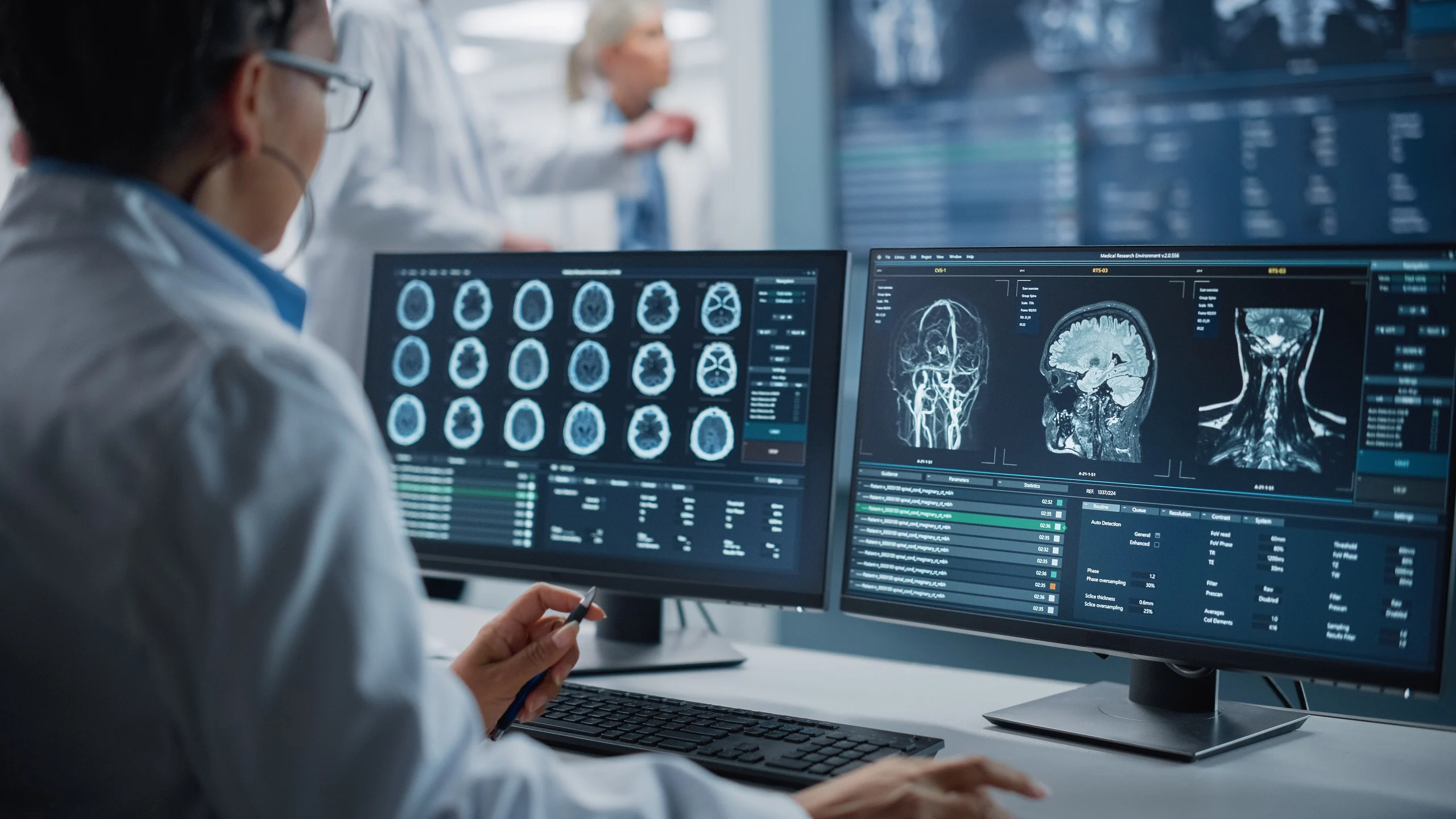
At the latest quarterly assembly of the Radiological Society of North America, prominent figures in modern imaging presented their state-of-the-art technology, drawing attention once again to artificial intelligence.
In a recent publication by the FDA, a comprehensive list was released in October encompassing machine vision systems and applications designed for analyzing MRI, CT, and X-ray images. The FDA has granted the majority of its hundreds of approvals for AI and machine learning-powered products in recent years to imaging tests.
Leading companies such as GE HealthCare, Siemens Healthineers, Philips, and Hologic are now striving to sustain this momentum. Over 90 programmers and companies are currently showcasing their innovations at the “AI Showcase” pavilion at the RSNA conference grounds within Chicago’s expansive McCormick Place convention center.
GE HealthCare, under the leadership of CEO Peter Arduini, aims to emphasize AI-integrated systems across its scanning, ultrasound, and online service portfolios to alleviate the burden on frontline healthcare providers by automating routine tasks and addressing the root causes of burnout.
Arduini emphasized the company’s commitment to providing timely information to healthcare providers, optimizing procedure efficiencies, facilitating quality care, and enhancing patient outcomes through intelligent devices, focused disease management, and digital solutions.
During the conference, GE HealthCare introduced its MyBreastAI Suite, a suite of technology tools for imaging procedures that includes three programs developed by iCAD. The company also highlighted its recent FDA clearance for automatic collapsed lung detection, underscoring its position as the 58th on the FDA’s list of AI approvals.
Meanwhile, Siemens Healthineers has embraced the concept of combining imaging scans with medical reports using generative AI, claiming to be the first in the industry to do so. The company envisions developing this technology into a chat program capable of providing tailored responses to professionals’ inquiries, leveraging dynamically generated and prioritized reviews based on clinical data, akin to systems like ChatGPT.
Philips, recognizing the challenges of physician fatigue with a reported 45% of radiologists experiencing long-term exhaustion, unveiled a cloud-based version of its Vue picture archiving and communication system (PACS) known as HealthSuite Imaging. This software aims to facilitate rapid adoption of new AI capabilities by pharmacists and clinicians for remote medical image analysis. Additionally, Philips introduced AI Manager, an integrated IT tool connecting staff with over 100 AI-based process programs.
Hologic announced the integration of its Genius AI Detection 2.0 deep learning system into its mammography program, with a goal of reducing false-positive breast cancer diagnoses by over 70% compared to its previous ImageChecker CAD software.
Philips also revealed the world’s first fully sealed smart MRI system as part of its “helium-free” portfolio, addressing concerns related to helium usage in traditional MRI systems. The BlueSeal MR Mobile 1.5T system, mounted on a truck, aims to provide imaging services to rural and underserved communities while addressing sustainability and operational cost challenges.
Siemens Healthineers outlined plans to enhance its Naeotom Alpha photon-counting CT system with features like respiratory scanning and imaging of patients with significant anatomical structures or stents. The company also disclosed its investment of €80 million ($86 million) to expand photon-counting detector production at its facility in Forchheim, Germany, with a focus on advancing CT technology for precise medical indications.
GE HealthCare introduced the future SIGNA Champion, a wide-bore 1.5T MRI scanner designed to offer a faster and more comfortable scanning experience, particularly for patients with positional challenges or specific health conditions. The organization aims to provide effective AI software tools alongside the SIGNA Champion, although approvals are pending in both the United States and Europe.